Introduction: Pediatric acute myeloid leukemia (AML) is a molecularly heterogeneous group of diseases lacking therapy options that would improve overall survival. Venetoclax (VEN) is an oral inhibitor for selective targeting of B-cell lymphoma 2 (BCL2), which is highly expressed in most patients (pts) with AML and has demonstrated promising efficacy in pediatric pts with AML when combined with chemotherapy (CTx) (Karol SE, et al. Lancet Oncol. 2020;21:551-560). Here, we present safety, efficacy, and preliminary genomic results from pediatric pts with relapsed/refractory (R/R) AML receiving VEN + CTx.
Methods: This phase 1 open-label, 2-part, multicenter study (NCT03236857) enrolled pts <25 years with R/R malignancies; here we report on R/R AML. During VEN monotherapy (monoTx), 10 pts received a weight- or age-adjusted adult-equivalent oral daily dose of 800 mg VEN after a 3-day ramp-up to mitigate tumor lysis syndrome risk. Standard of care CTx could be added after 21 days of VEN monoTx at the discretion of the treating physicians. In cohort expansion, 26 pts were enrolled with standard of care CTx allowed after VEN ramp-up. Primary and secondary endpoints included safety and preliminary efficacy of VEN monoTx and VEN + CTx. In addition, exploratory biomarker analyses were performed. For genomic analyses, whole exome sequencing was performed on pretreatment blood or bone marrow samples, and highly recurrent genetic alterations across various functional classifications in AML were analyzed. Previously documented gene fusion data were acquired through site-reported cytogenetic entries at screening. RNA sequencing was performed on pretreatment blood or bone marrow samples and BCL2 family expression was assessed.
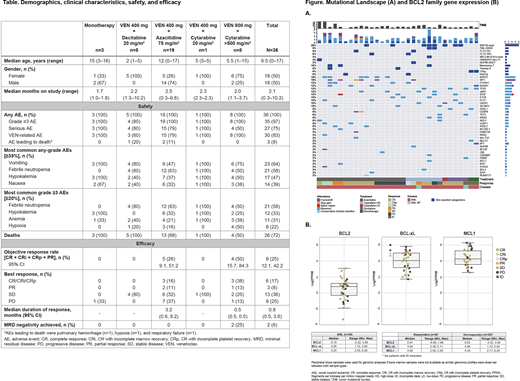
Results: As of June 2020, 36 pts with R/R AML were enrolled and received VEN monoTx (n=3) and VEN + CTx (n=33: VEN + decitabine [VEN-DEC, n=5], azacitidine [VEN-AZA, n=19], or low- [VEN-LDAC, n=1] or high-dose cytarabine [VEN-HDAC, n=8]) (Table). The primary reason for VEN discontinuation was progressive disease (n=19); median duration of VEN therapy was 3.1 months (range 0.2-9.3). All pts experienced adverse events (AEs); 3 pts (n=1 VEN-DEC, n=2 VEN-AZA) had fatal AEs considered unrelated to VEN. The most common grade 3/4 AEs were febrile neutropenia (58%) and hypokalemia (33%).
The overall objective response rate (ORR) was 25% (9/36); median duration of response was 0.8 month (95% CI, 0.5, 3.6). The best ORR was seen with VEN-HDAC (4/8, 50%) with 1 complete response (CR), 1 CR without platelet recovery, 1 CR with incomplete marrow recovery (CRi), and 1 partial response (PR); 2 pts achieved minimal residual disease negativity and 2 pts proceeded to transplant. The ORR with VEN-AZA was 26% (5/19), with 3 CR/CRi and 2 PR. No responses were seen with VEN monoTx or VEN + other CTx.
The genomic landscape of biomarker-evaluable pts was highly heterogeneous (Figure A). Mutations of genes involved in epigenetic modification (MYH11, IDH2, ASXL1, SETBP1, TET2, and NSD1) and transcription regulation (GATA1, WT1, RUNX1, and CEBPA) were the most common, in 58% and 48% of pts, respectively. Analysis of the recurring mutations found in ≥2 pts revealed that responses to VEN-AZA were seen in pts with IDH2 (1/4), MYH11 (2/6), RUNX1 (1/3), or FLT3 (1/3) mutations, and responses to VEN-HDAC were seen in pts with JAK2 (1/4) or GATA1 (1/3) mutations. Pts with WT1 (3/6) and PTPN11 (3/4) mutations responded to both regimens. Pts with TP53 (n=2) or ETV6 (n=3) mutations and PML-RARA (n=2) or KMT2A rearrangements (n=8) did not respond to any treatment. Gene expression profiling revealed that BCL-xL expression was significantly higher compared with BCL2;MCL1 levels were the highest (Figure B). There was no association between expression of these genes and response. Mutations were seen in BCL2 and MCL1 (n=1 each), but not in BCL-xL.
Conclusions: VEN + CTx was well tolerated in pediatric pts with R/R AML, with no unexpected toxicities. Preliminary efficacy was seen in pts receiving VEN-AZA or VEN-HDAC: ORR 26% and 50%, respectively. VEN + CTx resulted in responses in pts harboring mutations across a range of functional classifications; however, some alterations may confer resistance. Due to the limited number of pts harboring each mutation and the overall heterogeneity of the genomic landscape, these findings need to be evaluated in a larger population, and warrant further investigation.
Karol:AbbVie Inc.: Other: Unrelated to this study, St. Jude has received a charitable contribution from AbbVie, Inc. The charitable contribution is not being used for clinical or research activities, including any activities related to this study. . Bittencourt:Jazz Pharmaceuticals: Consultancy, Other: travel, accommodations, expenses; Novartis: Consultancy. Morgenstern:EUSA Pharma: Consultancy, Other: travel support; Bayer: Consultancy; Clarity Pharmaceuticals: Consultancy; BMS: Other: Institutional Research Funding; Boehringer Ingelheim: Consultancy; Roche: Consultancy. Macy:Merck: Other: Institutional Research Funding; Pfizer: Other: Institutional Research Funding; Bayer: Other: Institutional Research Funding; AbbVie Inc.: Other: Institutional Research Funding; Roche: Other: Institutional Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company. Khaw:Amgen: Other: Institutional Research Funding; Bristol-Myers Squibb: Other: Institutional Research Funding; AbbVie Inc.: Other: Institutional Research Funding; Novartis: Other: travel, accommodation, expenses; Walter and Eliza Hall Institute of Medical Research.: Patents & Royalties: Recipient of a share in royalty payments . Cooper:Celgene: Other: Spouse was an employee of Celgene (through August 2019). Ramsingh:Genentech: Current Employment, Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Tong:AbbVie, Inc.: Current Employment, Other: may hold stock or other options. Unnebrink:AbbVie: Current Employment, Other: may hold stock or other options. Vishwamitra:AbbVie, Inc.: Current Employment, Other: may hold stock or other options. Dunbar:Abbvie: Current Employment, Current equity holder in publicly-traded company. Prine:AbbVie: Current Employment, Other: may hold stock or other options. Palenski:AbbVie: Current Employment, Other: may hold stock or other options. Place:Novartis: Consultancy, Other: Institutional Research Funding; AbbVie: Consultancy.
Venetoclax is a BCL-2 inhibitor that is FDA approved for some indications. Venetoclax for treatment of pediatric AML is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal